[elfsight_social_share_buttons id=”1″]
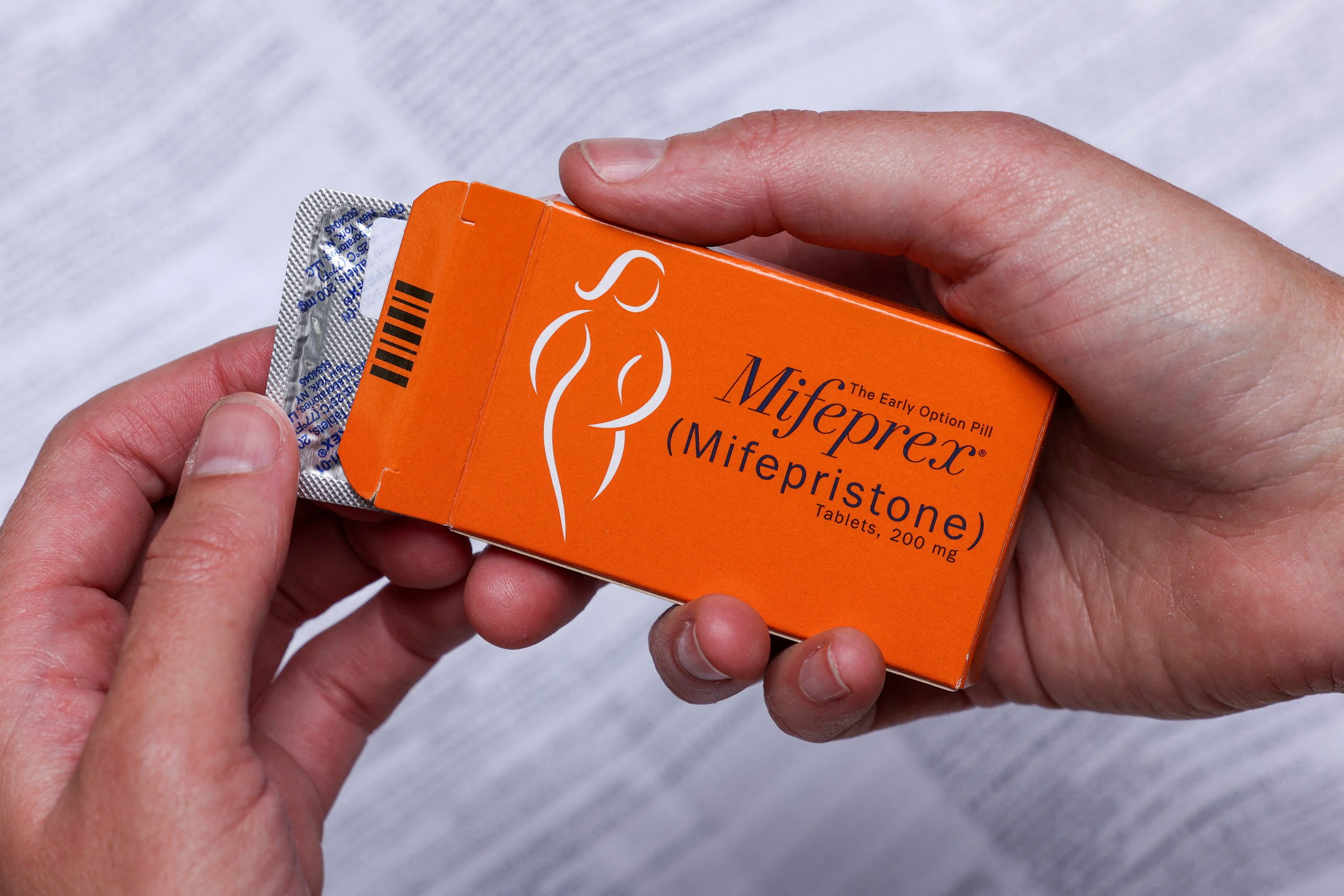
Twelve Democratic-led states have sued the U.S. Food and Drug Administration to challenge certain restrictions imposed on the distribution of the abortion pill mifepristone, saying those limits are not supported by evidence.
The lawsuit, led by Washington state and Oregon, was filed on Thursday in federal court in Yakima, Washington. A separate lawsuit by anti-abortion activists seeks to end access to the drug altogether.
Mifepristone, in combination with the drug misoprostol, is approved by the FDA for medication abortion in the first 10 weeks of pregnancy. Medication abortion accounts for more than half of U.S. abortions.
The new lawsuit challenges restrictions on mifepristone including requirements that doctors who prescribe it, and pharmacies that dispense it, obtain a special certification.
The FDA did not immediately respond to a request for comment.
FISM News reported in January that while these abortion pills are marketed as a safe and effective means of ending an unwanted pregnancy, Preborn! noted that, between 2000 and 2018, 24 mothers died and 4,000 reported serious complications, including hemorrhaging and life-threatening infections, as a result of taking the medications.
Medication abortion has drawn increasing attention since the U.S. Supreme Court last year reversed its landmark 1973 Roe v. Wade ruling that had legalized abortion nationwide. The ruling enabled more than a dozen Republican-led states to adopt new abortion bans.
Copyright 2023 Thomson/Reuters. Additions and Edits for FISM News by Jacob Fuller and Chris Lange
