Lauren Dempsey, MS in Biomedicine and Law, RN, FISM News
[elfsight_social_share_buttons id=”1″]
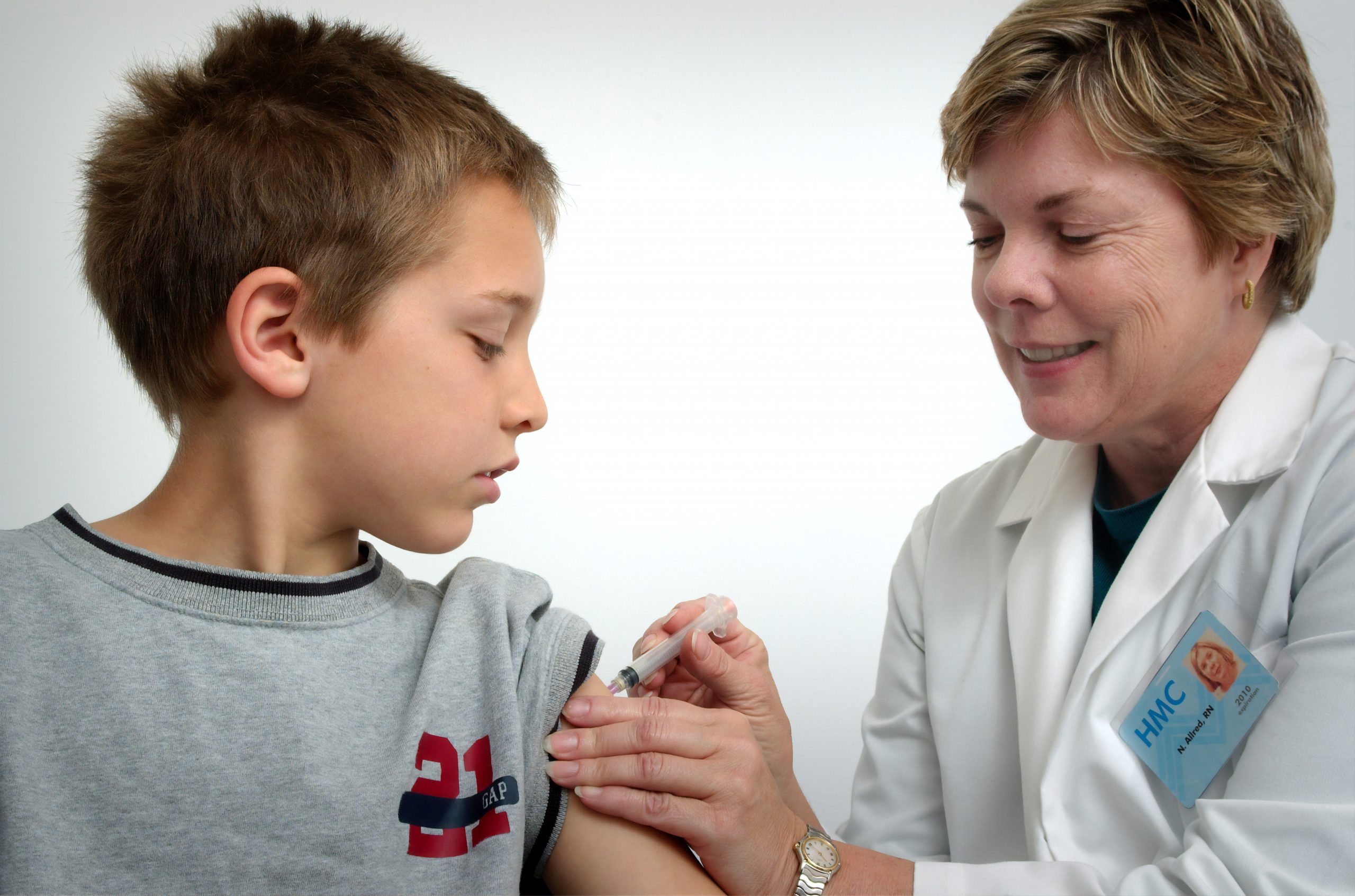
On Monday, Pfizer announced that it will be seeking FDA approval for the COVID-19 vaccine for use in children ages 5-11. The Pfizer vaccine is currently being administered under Emergency Use Authorization (EUA) in individuals aged 12-15, as well as for six month booster shots in those aged 65 and older or those that are immunocompromised.
The Pfizer BioNTech vaccine only has full FDA approval for use in individuals aged 16 and older.
But what’s the difference between full FDA approval and an EUA and why does it matter?
While clinical data that indicates treatments or medications are safe and effective are important in approval, an EUA is significantly different from FDA approval. This is because it is held to a lower standard, meaning that the FDA only requires reasonable evidence that a product may be effective.
The FDA can allow EUA approval before having all of the safety and efficacy information of a treatment, as well as before a medication has proven that the potential benefits outweigh the potential risks.
Just last week the Advisory Committee on Immunization Practices voted to reject Pfizer’s licensure in a vote of 2-16 for booster shots in individuals aged 16 and older. Dr. Phil Krause stated that much of the data that’s been presented and being discussed today is not peer-reviewed and has not been reviewed by the FDA. “The experts believe that the vaccines are doing what they are intended to do – prevent serious illness,” Krause said.
Pfizer revealed limited information in a press release on the clinical trial information that indicates safety and efficacy in vaccination of 5-11 year-olds. The trial has not yet been peer-reviewed, but it included over 2,000 children worldwide, aged 5-11. According to Pfizer the results suggest that “the vaccine was safe, well-tolerated and showed robust neutralizing antibody responses.” Children in the trial were administered a smaller dose of the vaccine, which appeared to be effective.
Unfortunately, the study was not large enough to identify any rare side effects, such as myocarditis. However, Pfizer’s senior vice president of clinical trials, Dr. Bill Gruber, stated that once the vaccine is authorized, children will continue to be monitored closely for rare side effects.
The trials are still ongoing and, as of now, there have not been enough positive cases of COVID in this age group to compare the vaccinated and placebo groups. Having this information will help to determine the ability of the vaccine to reduce severe illness in children. As a means of comparison, the initial clinical trial included over 43,000 adult participants.
Over 5 million children have tested positive for COVID-19 throughout the entire pandemic with rates declining. Even without vaccinations available to younger children, data reveals that they are at an extremely low risk for severe illness and death when compared to other age ranges. According to the CDC the hospitalization rates for children under 4 years old from the end of August are 2.4 per 100,000 children, which is 0.0024% and in children ages 5–11 years are 0.9 per 100,000, or 0.0009%.
Pfizer is also conducting Phase 3 clinical trials for children aged 6 months to 5 years old, with the goal that EUA approval for this age group will follow closely behind the approval for children aged 5-11. The data from this trial has not been released.
Vaccinating children is part of a larger push to reach herd immunity, which can also be obtained through natural immunity. If the FDA grants approval, millions of children will be eligible for vaccination by the end of October despite declining rates of hospitalization and severe illness and death in children.
