[elfsight_social_share_buttons id=”1″]
U.S. Supreme Court Justice Samuel Alito on Wednesday extended by two days a temporary block on limits set by lower courts on access to the abortion pill mifepristone in a challenge by pro-life groups to the drug’s federal regulatory approval.
The decision to keep the matter on hold gives the justices more time to consider an appeal by President Joe Biden’s administration and the pill’s manufacturer Danco Laboratories to block an April 7 preliminary injunction issued by U.S. District Judge Matthew Kacsmaryk in Texas that would greatly limit the availability of mifepristone while the litigation proceeds.
Alito’s order extended the pause on the dispute until Friday at 11:59 p.m. EDT. He had previously halted the lower court rulings until the end of Wednesday.
The Supreme Court has a 6-3 conservative majority.
The administration is seeking to protect the life-ending drug that now accounts for the majority of abortions now that the fight for the unborn has transferred primarily to the state level. Republican-led states have rushed to enact legislation that will restrict or ban abortions in their states following the Supreme Court ruling in June 2022 that overturned the landmark 1973 Roe v. Wade decision that legalized the procedure nationwide. Alito authored that ruling.
The White House is prepared for a long legal fight on the issue, Press Secretary Karine Jean-Pierre told reporters.
“We’re clearly keeping a close eye on this. … We are prepared for any outcome the Supreme Court may issue,” Jean-Pierre said.
The Food and Drug Administration (FDA), the U.S. agency that signs off on the safety of food products, drugs, and medical devices, approved mifepristone in 2000. The Texas court banned the drug, siding with plaintiffs who said that the drug did not go through the full-safety protocol of the FDA and discounted data showing that it had adverse side affects for women, including death.
“We will continue to stand with FDA’s evidence-based approval of mifepristone,” Jean-Pierre added. “… And we will continue to support FDA’s independent expert authority to review, approve and regulate a wide range of prescription drugs.”
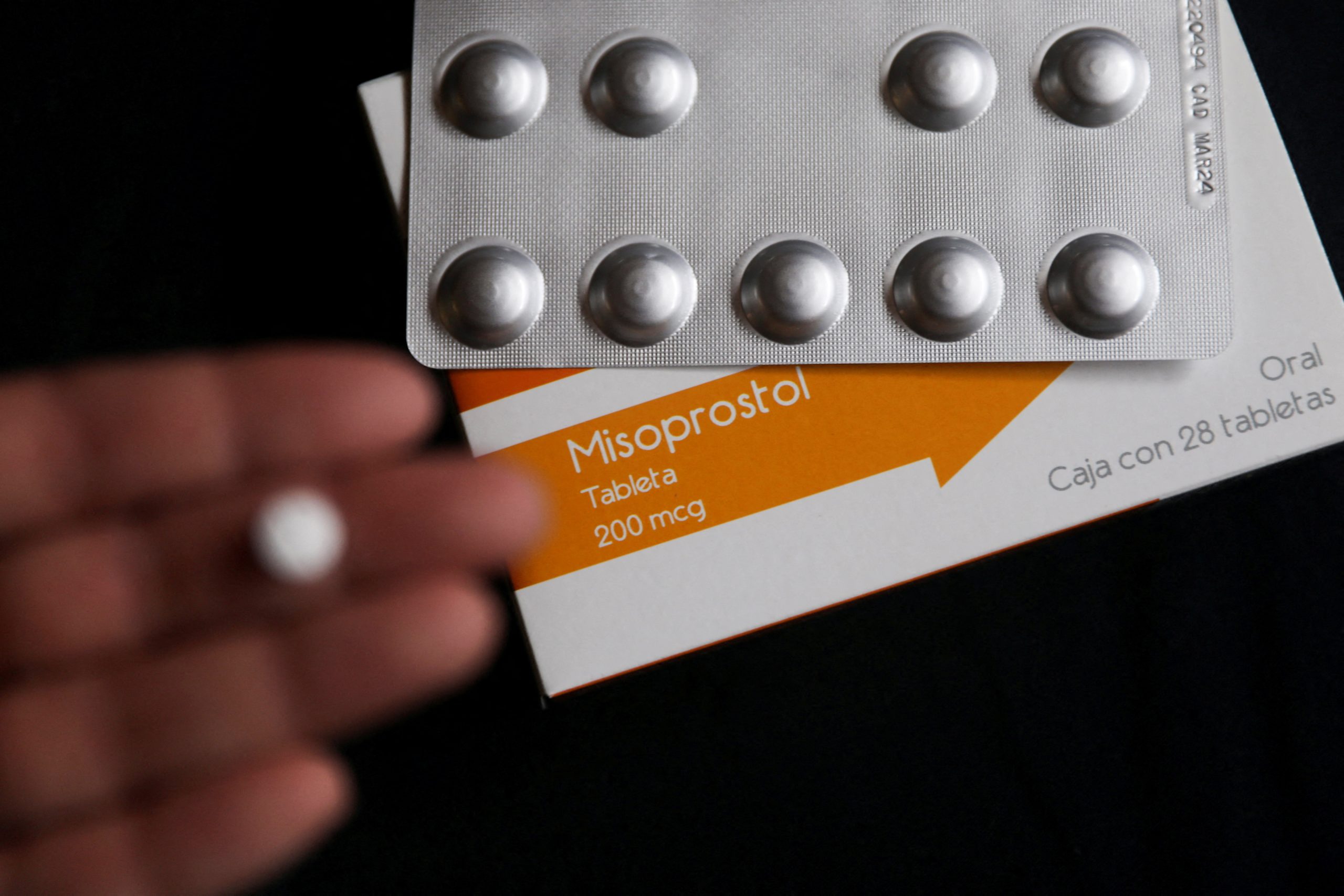
Mifepristone is used in combination with another drug called misoprostol to end the life of unborn children by denying them proteins needed to survive.
The administration and Danco told the justices in their filings that mifepristone might not be available for months if the restrictions were allowed to take effect.
The New Orleans-based 5th U.S. Circuit Court of Appeals on April 12 declined to block the curbs ordered by Kacsmaryk. The 5th Circuit did halt a part of Kacsmaryk’s order that would have suspended the FDA approval of the drug and effectively pull it off the market.
The restrictions, if enacted, would roll back actions taken by the FDA in recent years to make it easier to access mifepristone after confirming the pill’s safety and efficacy.
Those actions include in 2021 allowing mifepristone to be distributed by mail, and in 2016 approving its use up to 10 weeks of pregnancy instead of seven weeks, reducing the dosage required, and cutting the number of in-person doctor visits from three to one.
Copyright 2023 Thomson/Reuters. Additions and edits for FISM News by Michael Cardinal.
